The fight against cancer continues with “One More”
In the fight against cancer, “one more” matters - one more wedding, one more hug, one more trial. Healthcare professionals won’t stop fighting for their patients, and neither will we.
Learn More at Let’sOutdoCancer.com DetailsWe're Not Waiting for Breakthroughs - We're Working to Create Them
A cancer breakthrough isn’t one moment. It’s decades of research, millions of data points, and resilience in the face of setbacks – all with the hope of challenging what’s possible.
We’re not waiting for breakthroughs – we’re working to create them.
Learn More in USA Today Details
Advancing Cancer Care with Cutting-Edge Science
Powered by science and advanced technologies, we are committed to rapidly advancing novel combinations and next- generation biologics across a wide range of cancers – so that people with cancer can live better and longer lives.
Explore Pfizer Oncology DetailsProducts
Every product is the result of 1,500 scientists overseeing more than 500,000 lab tests and over 36 clinical trials before the first prescription.
Pfizer RxPathways
Pfizer RxPathways connects eligible patients to a range of assistance programs that offer insurance support, co-pay help, and medicines for free or at a savings.
Explore RxPathways Details
About
Starting with Charles Pfizer inventing an almond-flavored antiparasite medicine in 1849, our people have always been innovators and trailblazers, committed to finding the next cure.
Learn More About Us Details
Areas of Focus
Revolutionary medicines enable us to enrich and extend life for people living with all types of diseases.
An Accord for a Healthier World
Where people live shouldn’t impact the quality of their healthcare and income shouldn’t determine health outcomes.
Learn More About The Accord Details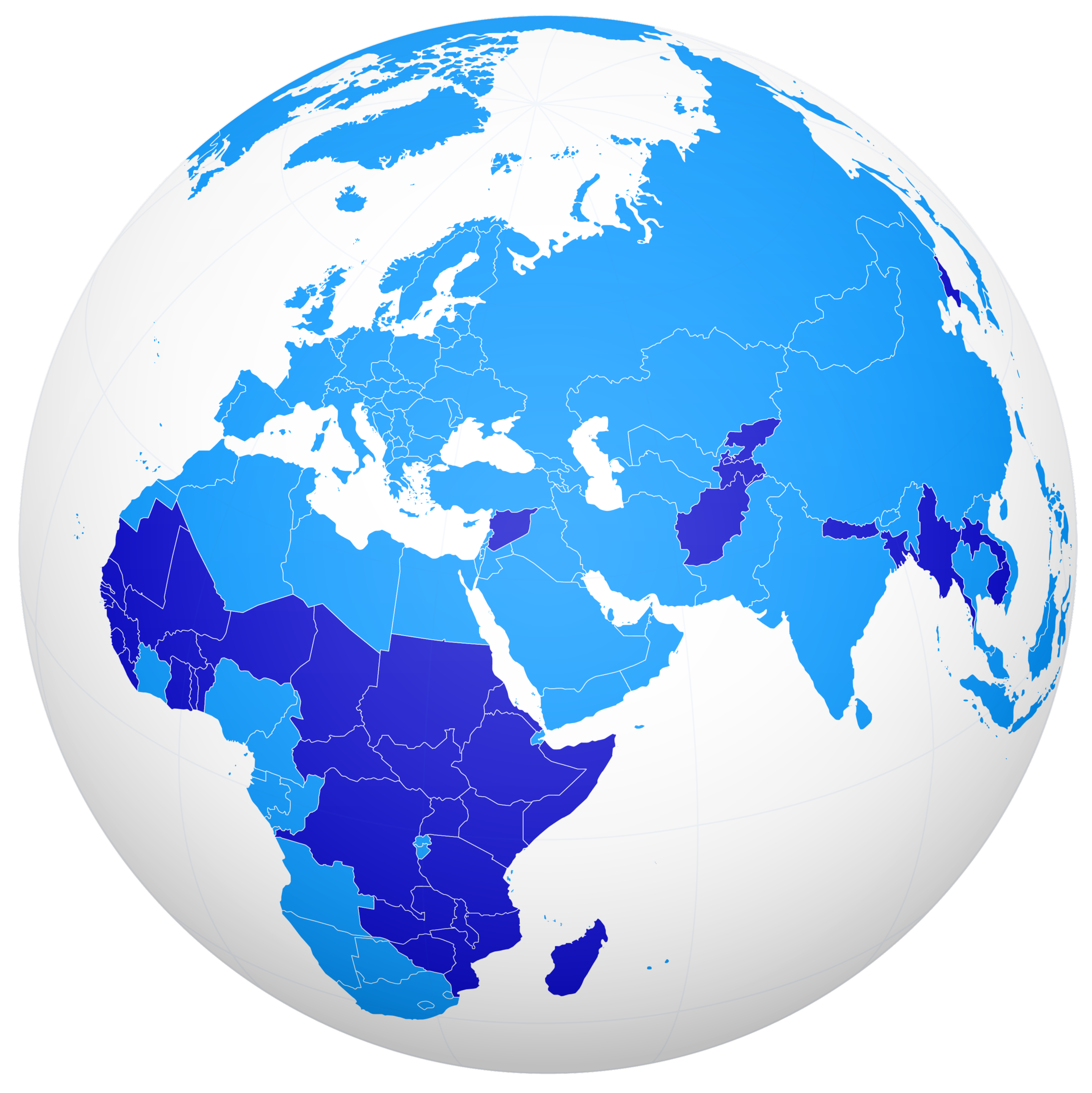
Sign Up for
communications from Pfizer
Receive the latest news from Pfizer in our monthly The Breakthrough newsletter and email alerts on a variety of topics.
Sign up now Details